Sunday, December 9, 2012
LED emission spectra
Thursday, November 22, 2012
The Pie in Pyrex
I was reading an article in the Fall2012/Winter2013 issue of "Chemical Heritage" (R. Blaszczyk, Cooking with Glass) about Pyrex bakeware. Apparently when Corning developed borosilicate ovenware, the first dish produced was a pie plate. The names considered for the glass were thus Pie-Rite or Py-Right, but then it was changed to Pyrex in 1915 (to rhyme with Nonex, another brand of Corning glass). Seems appropriate to mention pie on Thanksgiving! Hope yours is good.
Sunday, November 18, 2012
Starch-containing packing peanuts
Adding water to starch-based packing peanuts begins to dissolve them (conventional polystyrene peanuts are not soluble in water). If just a LITLLE water is added to the peanuts, only their edges dissolve and they beome sticky. The sticky peanuts can be then stacked into larger structures. Colored versions of these sorts of packing peanuts are marketed as "Nuudles". This demonstration illustrates concepts of like dissolves like, solvent welding, and recyclability of these starch-containing polymers.
Saturday, November 17, 2012
Hydrogen Balloon Fail
I was doing a demo show yesterday, and when my helper attempted to detonate a balloon containing hydrogen and oxygen gas with a lit candle on a stick, the ballon simply popped rather than detonating. This has happened a handful of time, typically with pure hydrogen in the balloons rather than a hydrogen-oxygen mix. What I think is happening is that when the candle flame approaches the balloon too slowly, its heat weakens the balloon and causes it to fail. The gases are then released in such a way that they do not detonate in contact with the frame.
Sunday, November 11, 2012
Fake Frost and Window Crystals
Saw this fake frost on the store windows at a Bass Pro Shop today. I do not know how it was made, but it shows pretty crystal patterns. For comparison, the van windows have real frost. One can saturate water with Epsom salt (magnesium sulfate) and paint the solution on dark-colored paper. When the water on the paper dries, the white salt crystals look like frost. When I was a graduate student some twenty years ago, I would occasionally work with organic compounds that melted at temperatures not far above room temperature. Sometimes a flask with a film of this melted liquid would cool in my hands. As the film solidified, pretty crystal stars would form along the walls of the flask. This was always fun to see. Update on 6-4-13: today one of my kids got some Crayola Crystal Effects Window Markers. When the marker ink dries it makes pretty crystal stars.
Friday, October 19, 2012
National Chemistry Week 2012
The theme for this year's 25th Annual National Chemistry Week (October 21-27, 2012) is nanotechnology. In the spirit of that I set up a display at Bradley University about nanotechnology and a ruler next to a sign saying "How tall are you in nanometers?" The idea is adapted from a display that I saw at the University of Texas-Austin last year.
Thursday, September 27, 2012
Analogy for Brownian motion
I was feeding a potato chip to a pool full of small fish and I noticed that as the little fish surrounded the chip and nibbled at it, the chip moved around randomly. I thought this was sort of an analogy for Brownian motion, where collisions between solvent molecules and small suspended particles causes the particles to move around in a random fashion.
Monday, September 17, 2012
Cover picture
The October, 2012, issue of the Journal of Chemical Eduation came out recently. I have two papers in it: one from work done at Bradley University "Take-Home Nanochemistry: Fabrication of a Gold- or Silver-Containing Window Cling", and the other from work at the University of Texas "New Nanotech from an Ancient Material: Chemistry Demonstrations involving Carbon-based Soot". A picture from the soot research was used for the cover.
Sunday, September 2, 2012
Sunday, August 26, 2012
Neil Armstrong's Passing
Just heard that Neil Armstrong passed away this past weekend. I was born about a month after his trip to the moon. Anybody who rides a rocket the size of the Saturn V has my respect - demos with small rockets are crazy enough. The picture was taken late last year at the Houston Space Center - very cool.
Friday, August 24, 2012
Beer Fluorescence
Wednesday, August 15, 2012
Gold Ruby Glass
Gold ruby glass (also known as cranberry glass) obtians its stunning red color from its structure at the nanoscale level. "Nanoscale" refers to the size scale range typically between 1-100 nanometers. A nanometer is very small (the prefix "nano" comes from the Greek word for dwarf) - it takes a billion nanometers to make a meter. For centuries, people have added gold compounds to glass, producing nanoscale gold metal particles - nanoparticles. These gold particles have the ability to absorb greenish light in a phenomenon called plasmon resonance. When white light, comprised of all colors, has greenish colors removed by the gold nanoparticles, the resulting light reaching the eyes appears red.
The photo was taken of a gold ruby glass collection at Bradley University (http://www.bradley.edu/about/publications/hilltopics/2012spring/lydia/).
Visible light spectroscopy was done on one of the plates (see: http://www.bradley.edu/inthespotlight/story/?id=96b71938-ee8a-4615-a06d-d04080208b2b). The spectrum is shown below:
A method for using gold compounds to produce gold nanoparticles in transparent silicone and give that spectacular gold ruby color can be found in D. J. Campbell, R. B. Villarreal, T. J. Fitzjarrald, “Take-Home Nanochemistry: Fabrication of a Gold- or Silver-Containing Window Cling” J. Chem. Educ., http://dx.doi.org/10.1021/ed200466k.

Visible light spectroscopy was done on one of the plates (see: http://www.bradley.edu/inthespotlight/story/?id=96b71938-ee8a-4615-a06d-d04080208b2b). The spectrum is shown below:
A method for using gold compounds to produce gold nanoparticles in transparent silicone and give that spectacular gold ruby color can be found in D. J. Campbell, R. B. Villarreal, T. J. Fitzjarrald, “Take-Home Nanochemistry: Fabrication of a Gold- or Silver-Containing Window Cling” J. Chem. Educ., http://dx.doi.org/10.1021/ed200466k.

Sunday, August 12, 2012
Sulfur in Illinois Coal
Illinois coal tends to have a bit of sulfur in it. Unfortunately, that makes it difficult to use as fuel for power plants, because it produces sulfur oxides, which in turn combines with water to produce acid rain. A story of how sulfur in coal damaged cars and clothes in Peoria, IL, is described in
D. J. Campbell, E. A. Wright,
M. O. Dayisi, M. R. Hoehn, B. F. Kennedy, B. M. Maxfield, “Classroom Illustrations of Acidic Air
Pollution Using Nylon Fabric”, J. Chem. Educ., 2011, 88, 387-391. The pictures show yellowish elemental sulfur and white crystalline sulfate salts on bituminous coal exposures near Bartonville, IL.
Friday, August 3, 2012
Finally found The Metric Marvels
For several years I have mentioned to people, including students in my classes, about cartoons that I saw as a little kid that were like School House Rock only they introduced me to the metric system. There was a Meterman (in the cartoon I learned that a millimeter was about the thickness of a dime and that a meter was a little longer than a yard) and there was a Wondergram (in the cartoon I learned that a gram was about the weight of a paper clip). Try as I might, I could not find out about these cartoons. I was beginning to think I had imagined the whole thing. Well, tonight I finally stumbled across information about them - they were called The Metric Marvels and more details can be found here: http://www.thefullwiki.org/The_Metric_Marvels. A black and white introductory clip can be seen here: http://www.youtube.com/watch?v=eW98QCk2FdA.
Sunday, July 22, 2012
Bubble Snouts
OK, not sure this is a demo so much as just a cool thing to do with bubbles. Simply use a rubber band to stretch terrycloth fabric over the wide part of a funnel made from the top of a 1 liter soda bottle. Dip the fabric into bubble solution and blow into the narrow part of the funnel (do not inhale!). The air pushes through the terrycloth to produce a stream or snout of many small bubbles.
Saturday, July 21, 2012
Geology Demo - Seltzer Popper Volcanoes
These demonstrations are a twist on the classic homemade volcano, in which vinegar and baking soda chemically react in a model of a volcano to produce sodium acetate, water, and carbon dioxide gas. The carbon dioxide bubbles up out of the model to produce bubbling “lava” which oozes out of the “volcano”. The picture below shows the same reaction in a soda bottle (food coloring and dish soap have been added to make the lava flow more impressive).


But what about the volcanoes with more explosive eruptions? Here we adapt another classic demonstration involving film canisters and Alka-Seltzer tablets. Wear eye protection. First lay down a sheet of plastic to make cleanup easier. Place a film canister into a clay volcano, setting the lid aside for now. Fill a milk cap with glitter. Fill the film canister halfway with water, with maybe a little dish soap and red and yellow food coloring for a “lava” effect. Break a tablet of Alka-Seltzer in half (any more that is wasted). Make sure everything is ready and the area is cleared out around the volcano.

Then, QUICKLY add the half-tablet of Alka-Seltzer to the film canister, cover the canister tightly with the lid, place the milk cap full of glitter on top of the lid, and take a step back (NEVER stand over the lid). The citric acid and baking soda in the tablet dissolve in the water and chemically react to produce sodium citrate, water, and carbon dioxide gas. The gas pressure builds up in the capped film canister until the lid pops off, scattering the glitter and sending reddish suds oozing down the side of the volcano.

Then, QUICKLY add the half-tablet of Alka-Seltzer to the film canister, cover the canister tightly with the lid, place the milk cap full of glitter on top of the lid, and take a step back (NEVER stand over the lid). The citric acid and baking soda in the tablet dissolve in the water and chemically react to produce sodium citrate, water, and carbon dioxide gas. The gas pressure builds up in the capped film canister until the lid pops off, scattering the glitter and sending reddish suds oozing down the side of the volcano.


We can also use the Alka-Seltzer tablets to illustrate the effects that size has on processes. If the half-tablet that is added to the film canister is first broken into small pieces, it will dissolve much more quickly in water to react and produce carbon dioxide gas. The result is that, with all else equal, a popper volcano with the broken-up half-tablet will pop more quickly after loading than one with an intact half-tablet. This illustrates how processes can proceed more quickly at smaller scales. For example, many people are studying how making things really small (at the nanoscale) with lots of surface area can speed up chemical reactions.
Another note: We have also noticed that a film canister fits into the top of a milk jug with just a little widening (we also colored the jug piece with permanent markers). This seems to be very inexpensive way to make a waterproof volcano cone without having to use modeling clay.

Friday, July 20, 2012
Geology Demo - Liquid Nitrogen Base Surge and Downburst
CAUTION: Liquid nitrogen is very cold and presents a serious frostbite
hazard, especially if it gets trapped against your skin (e.g.in your clothing).
Additionally, gaseous nitrogen occupies more volume than the same quantity of
liquid nitrogen. Gaseous nitrogen produced quickly enough in sufficient
quantities can displace oxygen from the air. Containers filled with liquid
nitrogen could fail without warning due to thermal shock or gas pressure.
Protect yourself accordingly.
Base surges and downbursts occur when dense gases sink rapidly down through
less dense gases and spread outward upon impacting the ground. Base surges are
clouds of gas and dust that move along the ground away from a volcanic eruption
or a nuclear explosion. It is often produced when a column of these clouds
collapses to the ground. Downbursts, and smaller microbursts, occur when cold
air sinks to the ground from a thunderstorm. A similar phenomenon seems to occur
during the filling of a typical 4 liter Dewar flask with a relatively narrow
neck from a liquid nitrogen supply line. As the liquid nitrogen (boiling at 77
K) first passes through the supply line and into the flask at room temperature
(about 298 K), the liquid nitrogen flashes into pressurized vapor. The vapor
rushes out of the Dewar flask, creating a column of cool nitrogen vapor and
condensed water droplets. As the supply line cools, liquid nitrogen actually
makes it into the Dewar flask. The liquid nitrogen boils away less rapidly and
the pressure forcing the nitrogen vapor up from the Dewar flask decreases. The
cold dense vapor column then collapses down and spreads across the ground,
resembling a base surge or a downburst.
References:
Clark Johnson. Base Surge.
http://www.geology.wisc.edu/~g111/Terms/base_surge/base_surge.htm (accessed
June, 2006).
Denton County, Texas. Downbursts.
http://www.co.denton.tx.us/dept/main.asp?Parent=82&Link=84#Mistaken
(accessed June, 2006).
BELOW: A liquid nitrogen base surge: (FIRST) Formation of the column of cool
nitrogen vapor and condensed water and (MIDDLE) collapse of the column. (LAST) Surge of air moving out from a thunderstorm rain column near Carlsbad, NM, in June, 2012.
Friday, July 13, 2012
Geology Demo - Liquid Nitrogen Geyser
Liquid nitrogen geysers have been observed on Neptune's moon Triton (these are believed to erupt via a different mechanism than here on Earth). A small liquid nitrogen geyser can be made by placing a meter-long copper tube partially into a pool of liquid nitrogen. The tube at room temperature is inserted into the Dewar flask that contains some liquid nitrogen. Since the boiling point of liquid nitrogen is 77K and the tube is approximately room temperature (298 K), liquid nitrogen entering the tube will quickly vaporize, pushing vapor and liquid nitrogen up the tube into the air for a brief time.


Wednesday, June 13, 2012
Faraday's Candle Chemistry
On the bus this morning I finished reading a copy of Michael Faraday's "The Chemical History of a Candle" based on his famous lecture series over 150 years ago. What an amazing lecturer he was! I had not realized how far back some classic science demos go. My favorite quote: "We young ones have the perfect right to take toys and make them into philosophy, inasmuch as now-a-days we are turning philosophy into toys." (He was using a suction cup toy to demonstrate air pressure.) I have not discussed candles much in my lectures, but I have used campfires as examples.
Here is a reference:
Here is a reference:
Faraday, M. The Chemical History of a Candle, Sesquicentenary ed., James, F. A. J. L., Ed.; Oxford University Press: Oxford, U.K., 2011.
Monday, June 11, 2012
Geology Demo - Candle Wax Demo


Sunday, June 10, 2012
Geology Demo - Snack Layers

Tuesday, June 5, 2012
Paper Demonstrations
- Folded Chiral Paper Structures
- Under development. Patterns containing brief instructions on construction and use of these folded paper structures can be downloaded as PDF files from the Internet at: http://bradley.bradley.edu/~campbell/chiralpapersprings.pdf. You are welcome to contact Dean Campbell (http://bradley.bradley.edu/~campbell/campbell@bumail.bradley.edu) to give him feedback on use of this template.
Chemical Cootie Catchers- Patterns containing brief instructions on construction and use of these folded paper structures can be downloaded as PDF files from the Internet at: http://bradley.bradley.edu/~campbell/chemcootiecatchers.html.
- D. J. Campbell, K. C. Campbell, K. M. Campbell, "Chemical ‘Fortune Tellers’ or ‘Cootie-Catchers’", Chem13 News, March, 2011, 8-9.
- Chemical Paper Snowflake Cutouts
- This paper describes the use of flat paper cutouts with six-fold symmetry for modeling layers of atoms within solid structures. Stacking the cutouts in specific ways illustrates how the layers of the atoms are stacked in the solids. Additionally, these cutouts can be used to demonstrate types of deformation in metals, atomic force microscopy, and carbon nanostructures. These paper lattices can be used on an overhead projector for demonstration to an entire class, or they can be constructed and studied on individual bases by students. K. F. Robinson, P. N. Nguyen, N. Applegren, D. J. Campbell, “Illustrating Close-Packed and Graphite Structures with Paper Snowflake Cutouts” The Chemical Educator, 2007, 12,163-166.
- Poisson's Ratio Cutouts Flat, flexible lattices can be used to illustrate Poisson’s ratios of materials. These lattices can be produced from ordinary sheets of paper.
D. J. Campbell, M. K. Querns, "Using Paper Cutouts to Illustrate Poisson's Ratio." J. Chem. Educ., 2002, 79, 76.
- Graphite Cleavage to Graphene Demonstrated with a Deck of Cards
- "When a pencil makes a mark on paper, tiny sheets of clay and graphite are rubbed from the pencil “lead” onto the paper fibers. Recently, scientists have repeatedly split stacked layers of graphite apart (like cutting a deck of cards) to isolate single-atom sheets of graphite. These single sheets, called graphene, have thicknesses of only a third of a nanometer – much thinner than the smallest piece of pencil dust." See: http://www.nano.utexas.edu/resources/nano-at-home/.
Sunday, June 3, 2012
Chemiluminesent Redox Reaction
Saturday, June 2, 2012
Oxidation of Iron Filings in a Sealed Bottle


Thursday, May 31, 2012
Coin Batteries



Tuesday, May 29, 2012
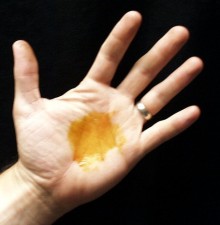
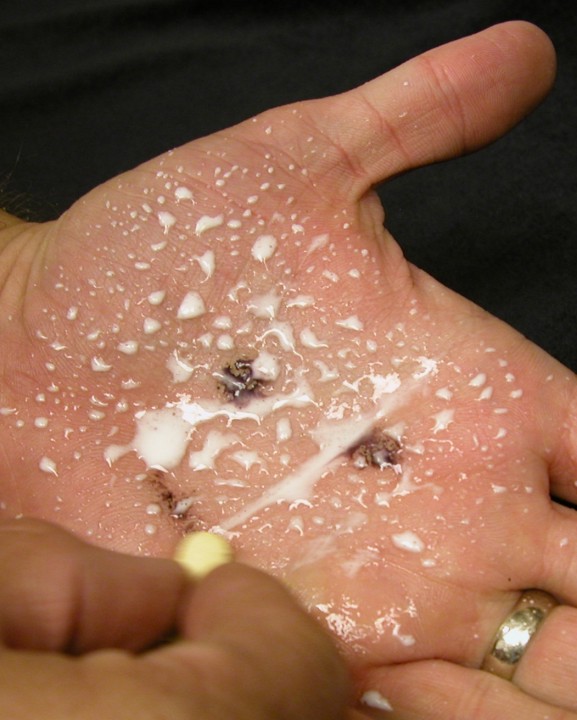
Vitamin C Redox Reaction Cleans Up Iodine Stains on Hand

Monday, May 28, 2012
Liquid Nitrogen Soap Suds Explosion


Sunday, May 27, 2012
Leidenfrost Effect with Liquid Nitrogen

Saturday, May 26, 2012
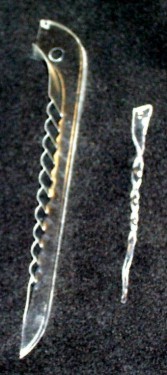
Homemade Shrinky Dinks®




Subscribe to:
Posts (Atom)







.jpg)
















