Sunday, August 26, 2012
Neil Armstrong's Passing
Just heard that Neil Armstrong passed away this past weekend. I was born about a month after his trip to the moon. Anybody who rides a rocket the size of the Saturn V has my respect - demos with small rockets are crazy enough. The picture was taken late last year at the Houston Space Center - very cool.
Friday, August 24, 2012
Beer Fluorescence
Wednesday, August 15, 2012
Gold Ruby Glass
Gold ruby glass (also known as cranberry glass) obtians its stunning red color from its structure at the nanoscale level. "Nanoscale" refers to the size scale range typically between 1-100 nanometers. A nanometer is very small (the prefix "nano" comes from the Greek word for dwarf) - it takes a billion nanometers to make a meter. For centuries, people have added gold compounds to glass, producing nanoscale gold metal particles - nanoparticles. These gold particles have the ability to absorb greenish light in a phenomenon called plasmon resonance. When white light, comprised of all colors, has greenish colors removed by the gold nanoparticles, the resulting light reaching the eyes appears red.
The photo was taken of a gold ruby glass collection at Bradley University (http://www.bradley.edu/about/publications/hilltopics/2012spring/lydia/).
Visible light spectroscopy was done on one of the plates (see: http://www.bradley.edu/inthespotlight/story/?id=96b71938-ee8a-4615-a06d-d04080208b2b). The spectrum is shown below:
A method for using gold compounds to produce gold nanoparticles in transparent silicone and give that spectacular gold ruby color can be found in D. J. Campbell, R. B. Villarreal, T. J. Fitzjarrald, “Take-Home Nanochemistry: Fabrication of a Gold- or Silver-Containing Window Cling” J. Chem. Educ., http://dx.doi.org/10.1021/ed200466k.
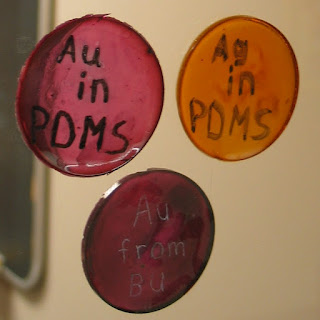
Visible light spectroscopy was done on one of the plates (see: http://www.bradley.edu/inthespotlight/story/?id=96b71938-ee8a-4615-a06d-d04080208b2b). The spectrum is shown below:
A method for using gold compounds to produce gold nanoparticles in transparent silicone and give that spectacular gold ruby color can be found in D. J. Campbell, R. B. Villarreal, T. J. Fitzjarrald, “Take-Home Nanochemistry: Fabrication of a Gold- or Silver-Containing Window Cling” J. Chem. Educ., http://dx.doi.org/10.1021/ed200466k.
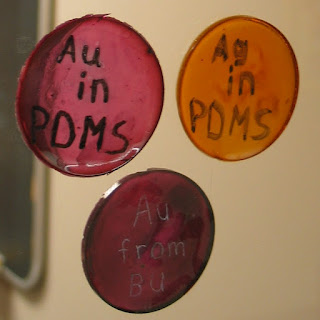
Sunday, August 12, 2012
Sulfur in Illinois Coal
Illinois coal tends to have a bit of sulfur in it. Unfortunately, that makes it difficult to use as fuel for power plants, because it produces sulfur oxides, which in turn combines with water to produce acid rain. A story of how sulfur in coal damaged cars and clothes in Peoria, IL, is described in
D. J. Campbell, E. A. Wright,
M. O. Dayisi, M. R. Hoehn, B. F. Kennedy, B. M. Maxfield, “Classroom Illustrations of Acidic Air
Pollution Using Nylon Fabric”, J. Chem. Educ., 2011, 88, 387-391. The pictures show yellowish elemental sulfur and white crystalline sulfate salts on bituminous coal exposures near Bartonville, IL.
Friday, August 3, 2012
Finally found The Metric Marvels
For several years I have mentioned to people, including students in my classes, about cartoons that I saw as a little kid that were like School House Rock only they introduced me to the metric system. There was a Meterman (in the cartoon I learned that a millimeter was about the thickness of a dime and that a meter was a little longer than a yard) and there was a Wondergram (in the cartoon I learned that a gram was about the weight of a paper clip). Try as I might, I could not find out about these cartoons. I was beginning to think I had imagined the whole thing. Well, tonight I finally stumbled across information about them - they were called The Metric Marvels and more details can be found here: http://www.thefullwiki.org/The_Metric_Marvels. A black and white introductory clip can be seen here: http://www.youtube.com/watch?v=eW98QCk2FdA.
Subscribe to:
Posts (Atom)



