Put wheels on a milk jug and alcohol vapor inside, then light the end and away it goes! Special thanks to Wayne Bosma at Bradley University for introducing me to the classic alcohol "whoosh" bottle. LEFT: The rocket car at the Bradley University High School Chemistry Contest in 2009. MIDDLE: The rocket car at
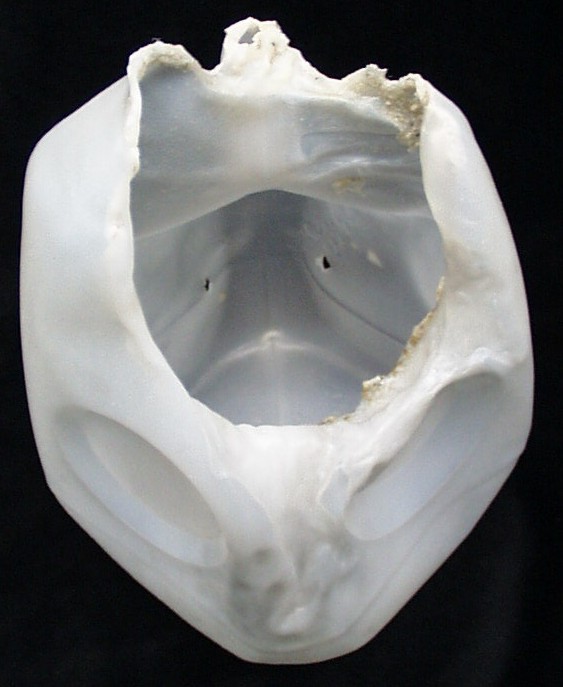
Demos on the Lawn '99 in Madison, Wisconsin. RIGHT: This melted jug might be what happens to the rocket car if you don't see the related paper in the
Journal of Chemical Education. I have recently developed a sturdier car construction than the one published in JCE.

 B
B
 D
D
 F
F G
G
 B
B
 D
D
 F
F G
G