Seltzer Popper Car
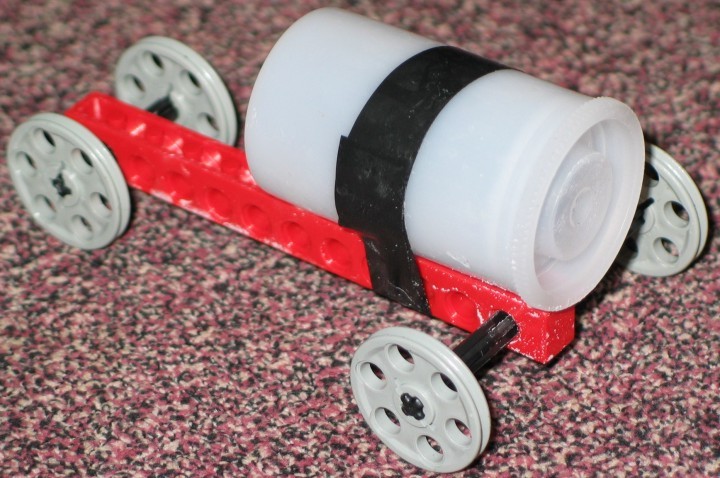
This is a rocket car that uses the pressure from chemical production of carbon dioxide as the basis for propulsion. The carbon dioxide is produced by reaction between acids and carbonate salts. The picture below shows a rocket car based on the popular demonstration involving water and AlkaSeltzer® in a 35 mm film canister placed on a chassis made from LEGO® parts. The "fuel" for the popping canister demonstration is approximately half an AlkaSeltzer® tablet, which is placed into a 35 mm film canister (Fuji-brand film canisters seem to work best). Water is added to the canister (to fill it approximately one third to one half full) and then the canister is capped. Ordinarily in the demonstration the canister is placed upright. The citric acid and the sodium hydrogen carbonate in the AlkaSeltzer® dissolve in the water and react to produce carbon dioxide. When pressure builds up, the cap pops a couple of meters into the air and spits out a quantity of fizzy water. The picture below shows the canister taped to a frame made of LEGO® parts. A popping canister will push a car made with this set of parts forward 10-20 cm. Other combinations of parts may produce cars that can move further. The challenge is to make a chassis that is lightweight, but with a wide wheel base to prevent tumbling. You are welcome to send alternative designs to me at campbell@bradley.edu. WARNING: The canister cap will fly backward with considerable speed, so a barrier to stop the cap is recommended. (The water will also spill out of the canister when it pops open.)
BELOW: A seltzer popper car.
 Thanks to Karen Campbell for passing information about this canister demonstration from an early childhood education conference to me and to Kristine Campbell for assistance with testing the car. More demonstrations involving LEGO® bricks may be viewed at the site "Exploring the Nanoworld with LEGO® Bricks."
Thanks to Karen Campbell for passing information about this canister demonstration from an early childhood education conference to me and to Kristine Campbell for assistance with testing the car. More demonstrations involving LEGO® bricks may be viewed at the site "Exploring the Nanoworld with LEGO® Bricks."



No comments:
Post a Comment